Its time for a clean break from “Muriatic Acid” and now there is a green alternative. This product is only made of US GOV FDA approved food additives. The US GOV FDA has already ran the toxicology on all our ingredients and they can be found in the US GOV FDA EAFUS listing of food additives. So our ingredients are just food or safe enough for ingestions, inhalation, eyes and skin. We are going to start with an overview of the risk and health concerns to you or your peoples (Eyes, Skin, Ingestion or inhalation) of workers, family members and children unnecessarily. The legal consequences of worker injury and permanent disability and even death to a company could be in the millions! The US GOV EPA and 3rd party have reported not only permanent damage to humans and the environment but even death. Part 1 is an overview for those that dont know of the health issues of Muratic Acid. Part 2 is examples of a technology being promoted by the US GOV USDA as part of the farm security act. In the federal government its mandatory to replace any chemical product Biobased or Bio-Preferred or Plant based ingredients. Best thing is all ingredients are grown by American farmers as its all just plant matter!
This is a list of uses of hydrachloric acid or Muriatic Acid for replacement. We are not stating that we can do these, but we do have a list of possible uses. We will have to go through a process of starting with known uses and over time add and deleting these other uses.
Acidize natural gas wells, Acidize petroleum oil wells, Brightens concrete, Brightens masonry, Brine treatment, Cement additive, Chemical production, Cleaner, Cleans metal, Cleans algae stains, Cleans Bathrooms, Cleans bricks, Cleans cement, Cleans concrete, Cleans fluxing, Cleans grout, Cleans hard surface, Cleans marble, Cleans masonry, Cleans mildew stains, leans mold stains, Cleans mortar, Cleans plasters, Cleans scam stains, Cleans steel, Cleans stone, Cleans tile, Cleans toilet bowl, Dichloroethane, Drinking water, Etching concrete, Ethylene dichloride, Fabrics processing, Food processing, Gold recovery, Ion exchangers, Masonry, Metals producing, Mineral processing, Mining operations, Molybdenum recovery, Non-oxidizing acid, Oil well acidizing, Ore processing, Organic chemical synthesis, Paints, Plastics, Poly vinyl chloride, Pool water, Potash ore production, Scale removal, Steel pickling, Surface oxides, Swimming pools, Tanning industry, Tungsten Metals, Uranium production, Varnish, Vinyl chloride, Wastewater treatment, Water treatment, Zirconium production
PART 1 The Green Alternative Safe Enough for a Baby’s Bottom!
So lets start with a overview of how we make our product. Its made as described below as just FDA EAFUS approved food additives. Safe enough to eat thats pretty simple to understand and it should give you a good feeling about product safety. So you might want to quickly read the overview from FDA of what EAFUS or (Everything Added To Food In the United States)

SGS Skin Test on 30 people proves its safe but we have known this for 17 years!

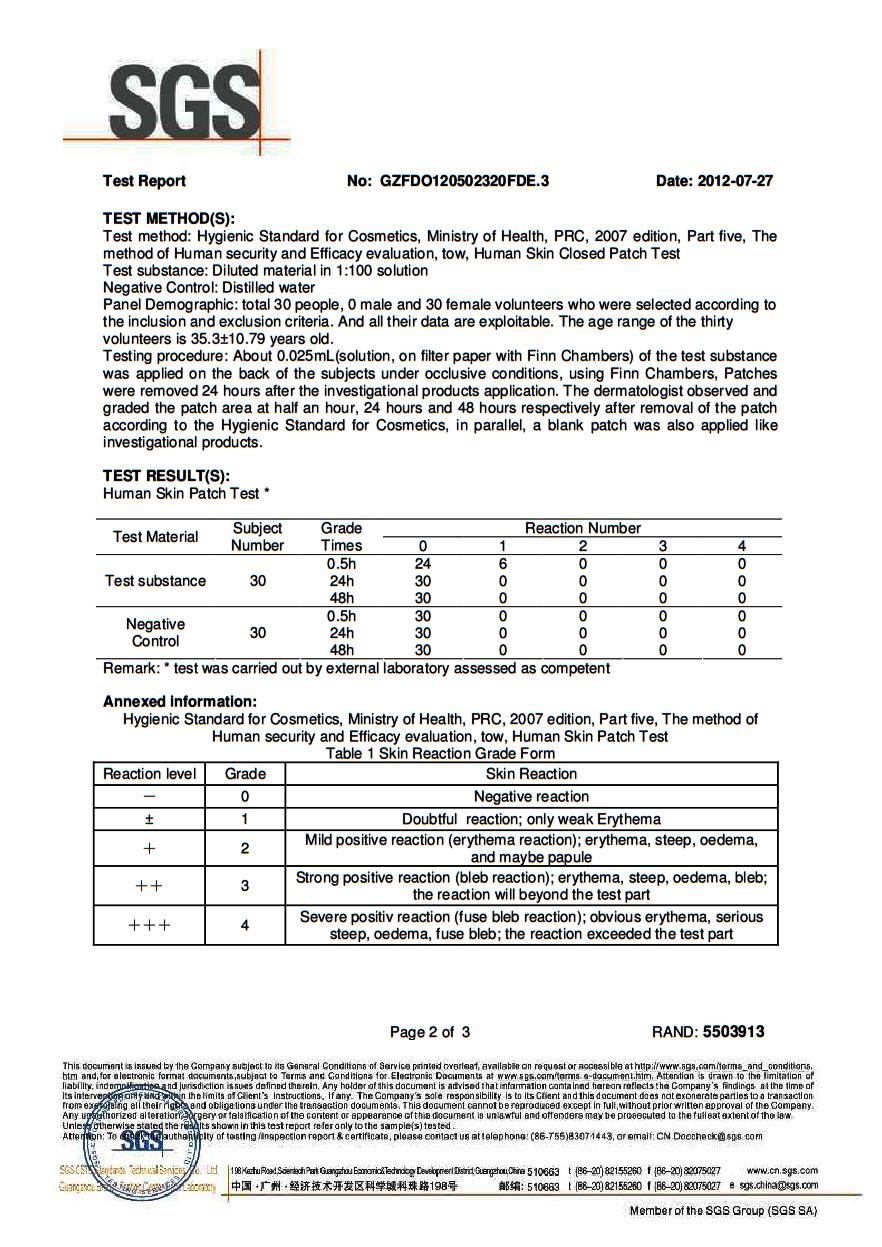

Now those SGS Tested Results Show No Skin Issues!
PART 2 Muratic Acid The Health Problem! Notice the Skull and Cross Bones below!


Twenty Milliion Tones of this Muraitic Acid are produced and used worldwide annualy. Who would ever want to use this product now that there is an alternative product which is a ecologically sustainable product made of farmer grown food additives. Why risk the health (Eyes, Skin, Ingestion or inhanlation) of workers, family members and children unnessarily. The legal consequnces of worker injury and permanent disability and even death to a company could be in the millions!

E
PA Report
Hydrochloric Acid (Hydrogen Chloride)
Hazard Summary-EPA Created in April 1992; Revised in January 2000
Hydrochloric acid has many uses. It is used in the production of chlorides, fertilizers, and dyes, in electroplating, and in the photographic, textile, and rubber industries. Hydrochloric acid is corrosive to the eyes, skin, and mucous membranes. Acute (short-term) inhalation exposure may cause eye, nose, and respiratory tract irritation and inflammation and pulmonary edema in humans. Acute oral exposure may cause corrosion of the mucous membranes, esophagus, and stomach and dermal contact may produce severe burns, ulceration, and scarring in humans. Chronic (long-term) occupational exposure to hydrochloric acid has been reported to cause gastritis, chronic bronchitis, dermatitis, and photosensitization in workers. Prolonged exposure to low concentrations may also cause dental discoloration and erosion. EPA has not classified hydrochloric acid for carcinogenicity.
Please Note: The main source of information for this fact sheet is EPA’s Integrated Risk Information System (IRIS), which contains information on inhalation chronic toxicity of hydrochloric acid and the Reference Concentration (RfC). Other secondary sources include the Hazardous Substances Data Bank (HSDB), a database of summaries of peer-reviewed literature, and the Registry of Toxic Effects of Chemical Substances (RTECS), a database of toxic effects that are not peer reviewed.
Uses
-
Hydrochloric acid is used in the production of chlorides, for refining ore in the production of tin and tantalum, for pickling and cleaning of metal products, in electroplating, in removing scale from boilers, for the neutralization of basic systems, as a laboratory reagent, as a catalyst and solvent in organic syntheses, in the manufacture of fertilizers and dyes, for hydrolyzing starch and proteins in the preparation of various food products, and in the photographic, textile, and rubber industries.
Sources and Potential Exposure
-
Occupational exposure to hydrochloric acid may occur via inhalation or dermal contact during its production and use.
Assessing Personal Exposure
-
No information was located regarding the measurement of personal exposure to hydrochloric acid.
Health Hazard Information
Acute Effects:
-
Hydrochloric acid is corrosive to the eyes, skin, and mucous membranes. Acute inhalation exposure may cause coughing, hoarseness, inflammation and ulceration of the respiratory tract, chest pain, and pulmonary edema in humans.
-
Acute oral exposure may cause corrosion of the mucous membranes, esophagus, and stomach, with nausea, vomiting, and diarrhea reported in humans. Dermal contact may produce severe burns, ulceration, and scarring.
-
Pulmonary irritation, lesions of the upper respiratory tract, and laryngeal and pulmonary edema have been reported in rodents acutely exposed by inhalation.
-
Acute animal tests in rats, mice, and rabbits, have demonstrated hydrochloric acid to have moderate to high acute toxicity from inhalation and moderate acute toxicity from oral exposure.
Chronic Effects (Noncancer):
-
Chronic occupational exposure to hydrochloric acid has been reported to cause gastritis, chronic bronchitis, dermatitis, and photosensitization in workers. Prolonged exposure to low concentrations may also cause dental discoloration and erosion.
-
Chronic inhalation exposure caused hyperplasia of the nasal mucosa, larynx, and trachea and lesions in the nasal cavity in rats.
-
The Reference Concentration (RfC) for hydrochloric acid is 0.02 milligrams per cubic meter (mg/m3) based on hyperplasia of the nasal mucosa, larynx, and trachea in rats. The RfC is an estimate (with uncertainty spanning perhaps an order of magnitude) of a continuous inhalation exposure to the human population (including sensitive subgroups) that is likely to be without appreciable risk of deleterious noncancer effects during a lifetime. It is not a direct estimator of risk but rather a reference point to gauge the potential effects. At exposures increasingly greater than the RfC, the potential for adverse health effects increases. Lifetime exposure above the RfC does not imply that an adverse health effect would necessarily occur.
-
EPA has low confidence in the study on which the RfC was based since it used only one dose and had limited toxicological measurements; low confidence in the database because the database does not provide any additional chronic or reproductive studies; and, consequently, low confidence in the RfC.
-
EPA has not established a Reference Dose (RfD) for hydrochloric acid.
Reproductive/Developmental Effects:
-
No information is available on the reproductive or developmental effects of hydrochloric acid in humans.
-
In rats exposed to hydrochloric acid by inhalation, severe dyspnea, cyanosis, and altered estrus cycles have been reported in dams, and increased fetal mortality and decreased fetal weight have been reported in the offspring.
Conversion Factors: To convert concentrations in air (at 25 °C) from ppm to mg/m3: mg/m3 = (ppm) × (molecular weight of the compound)/(24.45). For hydrochloric acid: 1 ppm = 1.49 mg/m3.
Health Data from Inhalation Exposure
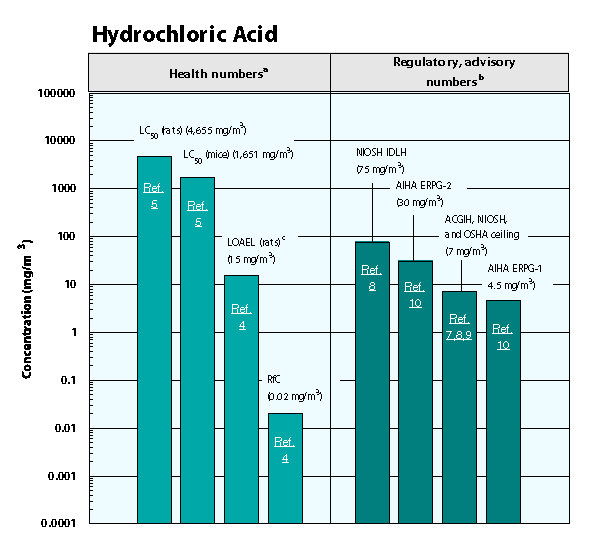
AIHA ERPG–American Industrial Hygiene Association’s emergency response planning guidelines. ERPG 1 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed up to one hour without experiencing other than mild transient adverse health effects or perceiving a clearly defined objectionable odor; ERPG 2 is the maximum airborne concentration below which it is believed nearly all individuals could be exposed up to one hour without experiencing or developing irreversible or other serious health effects that could impair their abilities to take protective action.
ACGIH TLV ceiling–American Conference of Governmental and Industrial Hygienists’ threshold limit value ceiling; the concentration of a substance that should not be exceeded during any part of the working exposure.
LC50 (Lethal Concentration50)–A calculated concentration of a chemical in air to which exposure for a specific length of time is expected to cause death in 50% of a defined experimental animal population.
LOAEL–Lowest-observed-adverse-effect level.
NIOSH REL ceiling–National Institute of Occupational Safety and Health’s recommended exposure limit ceiling; the concentration that should not be exceeded at any time.
NIOSH IDLH — NIOSH’s immediately dangerous to life or health concentration; NIOSH recommended exposure limit to ensure that a worker can escape from an exposure condition that is likely to cause death or immediate or delayed permanent adverse health effects or prevent escape from the environment.
OSHA PEL ceiling value–Occupational Safety and Health Administration’s permissible exposure limit ceiling value; the concentration of a substance that should not be exceeded at any time.
The health and regulatory values cited in this factsheet were obtained in December 1999.
1). Health numbers are toxicological numbers from animal testing or risk assessment values developed by EPA.
2). Regulatory numbers are values that have been incorporated in Government regulations, while advisory numbers are nonregulatory values provided by the Government or other groups as advice. OSHA numbers are regulatory, whereas NIOSH, ACGIH, and AIHA numbers are advisory.
3). This LOAEL is from the critical study used as the basis for the EPA RfC.
3rd Party Reports not from the EPA!
Muriatic Acid Burn Injury Treatment
Muriatic acid is another name for hydrochloric acid, which is a highly corrosive, poisonous liquid produced when hydrochloride, a gas, is mixed with water. It’s used in various manufacturing processes (in photograph processing and tanning leather, for example), and as a toilet bowl antiseptic, according to the Missouri Department of Health. It can severely burn throat, stomach, nasal and skin tissue when inhaled, swallowed or spilled.
Skin Burns
Flush the contaminated areas with water. High concentrations of vapor or liquid can cause the skin to redden and blister. In extreme cases, it can trigger frostbite, kill tissue or deeply ulcerated burns, the North Carolina Department of Health warns. Flush affected skin with water for 15 minutes but don’t apply soap or rub it. Remove any clothing or jewelry that may have come into contact with the chemical and seek medical advice.
Eye Burns
Exposure to eyes can irritate and burn or cause swelling, tearing, blurred vision, sensitivity to light and blindness. Like skin, eyes should also be flushed with water for 15 minutes. Lift the upper and lower lids while doing so. If the victim wears contact lenses, make sure they are removed. Seek medical advice if necessary.
Inhalation
Move the victim to fresh air and monitor respiration. Breathing in toxic amounts of this chemical will cause congestion, coughing and a burning sensation in the throat. Move the victim away from the accident scene into fresh air. Check for breathing irregularities and conduct CPR if necessary.
Ingestion
Force fluid intake and call for expert advice. Swallowing hydrochloric acid will quickly trigger severe pain in the mouth, throat, chest and abdomen, and can trigger nausea and vomiting. Get the victim to drink large amounts of water or milk to dilute the chemical’s strength, but do not force him to vomit unless told to do so by a physician. Call the National Capital Poison Center (800-222-1222) and seek immediate medical attention.
Types of Burns
Gauge the burn’s severity, if you’re capable of doing so before seeking direct medical intervention. It’s a good idea to check with a physician if any symptoms occur (and in all cases involving someone who’s swallowed hydrochloric acid). But minor exposure (short term, in small concentrations) to corrosive chemicals may only damage the top one or two layers of skin, causing first- or second-degree burns, many of which will heal on their own, according to the National Institutes of Health. In these cases, loosely covering the wound with sterile gauze after it’s been thoroughly flushed clean with water will help to protect it until the tissue heals. Third-degree burns (which penetrate all skin layers and the tissue underneath) can cause massive, permanent damage, tissue death and scarring. These burns need to be treated with topical and potentially oral or injected antibiotics to prevent possible serious infection. In extreme cases, it may be necessary to surgically replace lost tissue with skin grafts. If in doubt about the severity of the injury, consult a physician.
Muriatic Acid Dangers and What not to do!
Used for cleaning masonry, muriatic acid must be handled with care. A dilute form of hydrochloric acid, it can liberate both hydrogen and chlorine gases. Hydrogen is flammable and chlorine is highly toxic. Before using it, dilute further with water, adding the acid to a measured amount of water, never the other way around. Take steps to protect nearby metalwork and plants. When you’re finished, dispose of the unused acid by carefully neutralizing it with gardening lime or taking it to a hazardous waste handler.
Burns
Highly concentrated muriatic acid can burn skin and other body tissues. Always wear protective clothing, gloves and eye wear when handling it.
Dilution
When diluting muriatic acid with water, don’t add water to acid. The mixture may erupt, spraying you with acid. Instead, always add acid to water. Metals
Muriatic acid
Can corrode and weaken metals. When cleaning with it, protect any nearby structural metalwork with plastic.
Vapors
The mist and vapors from muriatic acid are also hazardous. If you use it, make sure there’s plenty of ventilation. Avoid using it on days when the wind can carry it.
Chlorine Gas
If you find an old, half-filled bottle of muriatic acid, don’t open it. The chlorine in the acid will, over time, evaporate from the liquid into the empty space. Chlorine gas is reactive and toxic. Take the bottle to a hazardous waste handler.
The Effects of Hydrochloric Acid on Humans of Inhaling Muriatic Acid Fumes
Ingestion
Acute oral exposure to muriatic acid via ingestion causes severe burns of the mouth, mucous membranes, esophagus, and stomach. Symptoms that may be experienced range from mouth, throat, chest and abdominal pain to breathing difficulties due to swelling of the throat, drooling, fever, vomiting blood and rapid drop in blood pressure. If the person who ingested a muriatic acid solution is conscious, provide large amounts of milk or water to dilute the solution. Do not induce vomiting. Seek immediate medical attention.
Inhalation
Short term inhalation exposure to muriatic acid causes eye, nasal, and respiratory tract irritation and inflammation, as well as pulmonary edema. Brief exposure may be lethal. Symptoms exhibited after muriatic acid exposure include a bluish tinge to lips and fingernails, tightness in the chest, shortness of breath, coughing, choking, vertigo, rapid pulse, low blood pressure and weakness. Move a person affected by inhalation of muriatic acid to fresh air at once. Keep them warm and perform CPR if breathing stops. Seek immediate medical attention.
Eye and Skin Exposure
Muriatic acid skin and eye contact produces severe burns, ulceration, and scarring. The severity of the burns depends directly on the strength of the solution. Severe burns may progress to ulcerations that lead to scarring. Long-term skin contact causes dermatitis. Contact with eyes may cause reduced vision, cataracts or blindness. Flush affected skin areas or eyes with water for a minimum of 15 minutes. Do not rub or wash skin. If clothing has been penetrated, remove it prior to flushing the skin with water. Seek immediate medical attention.
Muriatic Acid Precautions
Muriatic acid, the historical name for hydrochloric acid, is a clear, colorless, mineral acid that is useful for laboratory research and industrial manufacturing processes. It is, however, a very poisonous, corrosive liquid that reacts with a variety of materials and can severely damage body tissue and organs. The concentrated acid produces toxic fumes. The acid should therefore be stored and handled with care.
Chemical Composition and Reactivity
Muriatic acid is a solution of hydrogen chloride (HCl) gas in water, and its chemical formula is often written as HCl. It is classified as a “strong” acid because, in water solution, it is almost entirely dissociated into H+ and Cl- ions. The H+ ions are normally associated with water molecules to form hydronium (H3O+) ions. The acid reacts with many substances, including bases, metals, metal oxides and hydroxides, carbonates and amines. If decomposed by heat, the acid produces toxic chlorine gas and highly flammable hydrogen gas.
Physiological Hazards
If muriatic acid comes in contact with the skin, it can cause reddening, pain, severe burns and ulcers. Contact of the acid or its vapors with the eyes can irritate, burn and damage the eyes. Swallowing the acid immediately burns the inner linings of the mouth, throat and digestive tract, and may lead to nausea, vomiting and even death. If the acid vapors are inhaled, they can cause coughing, choking and inflammation of the upper respiratory tract; in extreme cases, inhalation can lead to accumulation of fluid in the lungs (pulmonary edema) and death.
Workplace Safety
Storage: Muriatic acid should be stored in a cool, dry area, away from heat, moisture and direct sunlight. The room should be well-ventilated, equipped with a good drainage system and have acid-resistant floors.
Handling and Disposal: To prevent contact with the skin and clothing, wear rubber or neoprene gloves, a protective apron and impervious shoes or boots. To protect the eyes, wear safety goggles or a full facial shield. When diluting the acid, add the acid carefully and in small quantities to water. Do not add water to the acid, as that can generate extreme heat and may lead to boiling and splashing. When disposing of the acid, treat it as hazardous waste and send it to an approved waste facility.
First Aid: If muriatic acid contacts the skin or eyes, use plenty of water to flush the areas of contact, over a period of at least 15 minutes. Remove any contaminated clothing. If a person has ingested the acid, give the patient large quantities of water or milk to drink, but not if the patient is unconscious; do not induce vomiting. If someone has inhaled vapors of the acid, bring the person into fresh air. In case of breathing difficulty, provide oxygen; in case breathing has stopped, provide artificial respiration. Immediately call for medical attention.
Cleaning a Leak or Spill: In case of an accidental leak or spill of muriatic acid, cordon off the area, ventilate it and keep unprotected people away. Wear protective gloves, goggles, apron and shoes, as noted above. Carefully neutralize the acid with soda ash (sodium carbonate) or lime (calcium oxide) and absorb the liquid with dry sand, earth or other noncombustible material. Place the material in a container for chemical waste and send it to an approved waste facility.

